
Gedeptin® is a gene-directed enzyme prodrug therapy (GDEPT) that results in the formation of the oncolytic agent within the tumor itself, resulting in tumor cell death while significantly limiting systemic exposure. Preclinical and early-stage human clinical data suggest the GDEPT approach can destroy otherwise refractory cancer cells in a safe and effective manner.
Gedeptin® is based upon a replication-deficient adenoviral vector, a well-recognized vaccine platform used in existing vaccines. Gedeptin® codes for an enzyme derived from E. coli called purine nucleoside phosphorylase (PNP). Gedeptin is injected directly into the tumor mass three times over a period of two days. The PNP enzyme, by itself, has no anti-cancer activity, and because it is derived from bacteria, the enzyme is unlikely to have significant metabolic activity in humans. However, the PNP enzyme can be used in combination with purine nucleoside prodrugs in order to generate active chemotherapeutic agents within the tumor cells (in situ).
The purine nucleoside drug, fludarabine phosphate (Fludara®), is approved for the treatment of certain hematological tumors but has shown minimal effectiveness against solid tumors. However, when fludarabine is taken up by cells previously treated with Gedeptin®, the absorbed fludarabine is converted, in situ, to the potent cytotoxic agent, fluoroadenine. A cycle of Gedeptin® therapy involves the intratumoral administration of three doses of Gedeptin® over a two-day period. This is followed by the intravenous administration of fludarabine phosphate once a day for the next 3 days. The result, when the Gedeptin-treated cells absorb the fludarabine, is the targeted, site-specific activation of fludarabine to the active “cancer-killing” chemotherapy, fluoroadenine, within the cancer cells themselves.
Fluoroadenine works by blocking DNA synthesis and is particularly effective at killing the rapidly dividing refractory solid tumors dependent on DNA, RNA, and protein synthesis for growth.

Gadeptin® has already been awarded U.S. FDA Orphan Drug status for the intratumoral treatment of anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of the mouth, salivary gland, and other oral cavities. The orphan drug designation is awarded to drugs designed to treat a rare disease or condition that affects fewer than 200,000 people in the U.S., and it is applied specifically to novel therapeutics that represent a major improvement. Orphan drug status provides regulatory incentives, reduced fees, and a more rapid review by the FDA and stipulates that competing therapies can be blocked from the market for up to seven years. Additionally, this status qualifies the drug sponsor for various development incentives, including tax credits for qualified clinical testing.

Gedeptin is in a Phase 1/2 trial being conducted at Stanford University, Emory University, and Thomas Jefferson University. The trial design involves repeat administration using Gedeptin, followed by systemic fludarabine, as a way to gain additional information prior to expansion towards a larger patient trial. The initial stage of the study (10 patients) is being funded by the FDA pursuant to its Orphan Products Grants Program. Five patients have been enrolled to date.
GeoVax is devoting resources toward facilitating the completion of patient enrollment for the initial stage of this study and expanding the trial to additional study sites resulting in 25-35 patients in total. We anticipate completion of the initial 10 patient studies yet this year, including our review of the results. We will review those results with the FDA along with our recommendations for an expanded program while also discussing with the FDA the potential for an expedited BLA filing.We believe that there is an opportunity to accelerate the path to registration should the current, expanded clinical trial result in significant improvement over the standard of care among the patients in the trial.

PNP Therapeutics conducted a first-in-human Phase 1 clinical study (NCT01310179) to evaluate Gedeptin for the treatment of refractory head and neck cancer. The study enrolled 12 subjects. Results published in the Annual of Oncology in 2015 (Rosenthal EL et al., 2015) show all 12 study subjects completed therapy without dose-limiting toxicity. Tumor size change from baseline to final measurement demonstrated a dose-dependent response, with 5 of 6 patients (83%) in the higher dose cohorts (cohorts-3 & -4) achieving significant tumor regression (see graphs below). One patient in cohort-4 (see image below) demonstrated noticeable improvement in tumor response after only one treatment cycle (14 days).
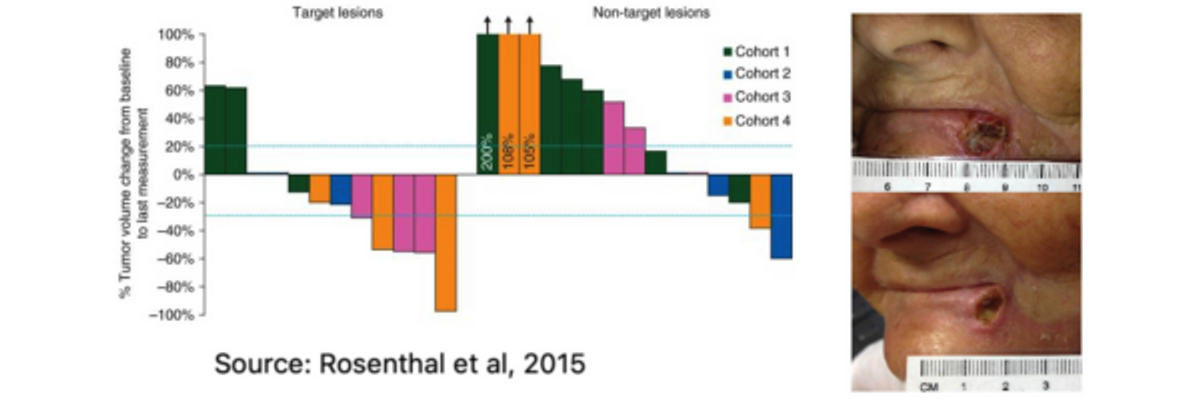
Notably, despite fludarabine’s known systemic toxic effects, the overall adverse event rate was not dose-dependent, and the vast majority of events were deemed mild or moderate in nature. In addition, there were no treatment-related serious adverse events, and analysis of patient serum confirmed the lack of systemic exposure to fluoroadenine and the PNP enzyme.

Head and neck cancer account for about 3-5% of all cancers in the United States. In 2009, the American Cancer Society estimated that 48,010 people developed head and neck cancer and there are an estimated 15,000 patients within the U.S. who die annually as a result of advanced head & neck cancers. Worldwide, there are an estimated 400,000 deaths annually from Head & Neck cancers. This represents our targeted patient population (those who die annually from head and neck cancers).

Patients with advanced head and neck cancer are often treated with a combination of radiation and chemotherapy in the front-line setting, along with immunotherapy. Patients with targetable mutations, such as HER2, EGFR, or Trop-2 may receive targeted therapy, but checkpoint inhibitors, such as Keytruda® (Merck), are now common for front-line with or without chemotherapy. Several pharmaceutical companies are working on combination therapy with checkpoint inhibitors to enhance response. These include DNA-damaging agents called PARP inhibitors, oncolytic viruses, cellular therapies, or cancer vaccines that target viral antigens in HPV+ patients.
GeoVax believes that Gedeptin holds potential as a single agent and in combination with checkpoint inhibitors or other targeted agents in both early-stage (front-line) and refractory patients.

Work continues with the company’s use of its modified Vaccinia Ankara (MVA) virus delivery vector to introduce tumor-associated antigens (TAA) designed to provoke the immune system. TAA are proteins, glycoproteins, glycolipids, or carbohydrates expressed on the surface of tumor cells that the immune system may use to identify a malignant cell from a healthy, normal cell. It is well known that many cancers have ways to turn off or hide from the body's ability to find and identify important TAAs. Herein is the challenge that GeoVax is addressing to overcome.
GeoVax initial focus to leverage its MVA platform is with Mucin 1 (MUC1). MUC1 is an oncogenic transmembrane glycoprotein in the mucin family found to be overexpressed in a wide variety of hematologic malignancies and solid tumors, including mesothelioma, breast, lung, gastric, pancreatic, colon, ovarian, and endometrial cancers. High expression correlates with aggressive, metastatic disease and poor response to conventional therapy (Horm & Schroeder, 2013). The company’s MVA-VLP-MUC1 candidate is designed to introduce the MUC1 antigen, which, much like a vaccine, activates the immune system.
Thus, GeoVax’s primary strategy is to combine its MUC1 vaccine with checkpoint inhibitors, such as Keytruda® or Opdivo® (Bristol-Myers), along with standard-of-care chemotherapy or radiation therapy. This triple-combination approach has the potential to unmask the MUC1 target and allow the body's own immune system to find and destroy cancerous cells with the help of broad-spectrum systemic care.
The company has recently presented results from two preclinical programs. In a therapeutic evaluation, MVA-VLP-MUC1, in combination with anti-PD-1, resulted in a 57% reduction in tumor growth compared to untreated controls. In a preventive (“tumor recurrence”) model, vaccination with MVA-VLP-MUC1 resulted in 100% prevention of tumor growth vs. 100% tumor growth in the untreated cohort.
Gedeptin® provides GeoVax with another pillar on which to build its immuno-oncology pipeline. Furthermore, the anticipated synergy between Gedeptin® and MVA-VLP presents additional opportunities for novel cancer therapies. For example, the MVA-VLP platform can be used to awaken the immune system, and Gedeptin can be used to achieve direct tumor cell oncolysis. Both agents can be combined with checkpoint inhibitors to reverse immune tolerance and drive long-lasting responses.
Over the past two years, GeoVax has expanded its infectious disease development pipeline to include programs targeting Coronavirus, including a COVID-19 vaccine for immunocompromised patients, a COVID-19 booster vaccine, and a pan-Coronavirus vaccine.
The company continues to focus on COVID-19 vaccine development because there are significant limitations to the first-generation vaccines that were deployed during the emergency authorization period in 2020 and 2021.
One of these limitations was the emergency of immune escape due to the first-generation vaccines relying solely on targeting the S-protein to mediate resistance and neutralizing capacity. The S-protein is highly variable and susceptible to point mutations that result in antigenic drift, which leads to the emergence of new variants of concern (VOC), such as the Delta and Omicron variants that reignited waves of infection and re-infection all over the world. Another limitation of the first-generation vaccines was the short duration of antibody response, also known as kinetics, and the lack of strong cellular (T-cell) response, or immunological memory, that limited long-term protection. This second issue is a particular area of concern for elderly patients or patients who are partially immunocompromised because they cannot mount nor maintain a high enough level of a protective response to the first-generation mRNA vaccines to convey long-term protection.
GEO-CM04S1 (“CM04S1”) is our next-generation COVID-19 vaccine designed to increase the magnitude, duration, and functional breadth of the immune response, including the establishment of long-term immunological memory to achieve a higher level of protection.

CM04S1 is a synthetic attenuated modified vaccinia Ankara (sMVA) vector expressing both the spike (“S”) and nucleocapsid (“N”) antigens of the SARS-CoV-2 virus - with each component playing a key role in the immune response. What makes this technology so interesting for COVID is that MVA has a large and available genetic coding capacity allowing for the insertion of multiple genes into different sites, supporting the simultaneous expression of multiple immunogenic proteins, such as the S and N proteins found on SARS-CoV-2. MVA also presents antigens through the cross-presentation pathway, which is highly effective for the induction of antibody and CD4+ T cell responses.
Our preclinical data show that CM04S1 induces antibody responses, similar to the mRNA vaccines, by targeting the S-protein, but by also targeting the N-antigen, CM04S1 drives a strong T-cell response, including T-cell memory. Targeting the N protein is particularly important because it is the most abundant protein in coronaviruses; it is highly immunogenic, and its amino acid sequence is largely conserved, which should protect against mutations in the S protein and provide a more robust and durable response (i.e., less opportunity for viral escape) in patients who failed to mount a high enough response to the first-generation mRNA vaccines.
Combined, this makes the CM04S1 vaccine ideal for immune-compromised patients and as a booster to approved mRNA vaccines. For the latter, mRNA vaccines are being proposed for fourth and fifth doses due to a combination of lack of long-term immunity, viral escape variants, and other factors. From a public health perspective, assuming large populations will get vaccinated 3-5+ times is just not practical.
Phase 1 data in The Lancet Microbe, demonstrates that GEO-CM04S1 produced robust neutralizing antibodies and T cells against SARS-CoV-2 with no significant side effects. In addition, the CM04S1 has demonstrated protection against VOC, including the Delta and Omicron variants. The takeaway here is that GeoVax’s vaccine offers a dual mechanism of action that should provide better protection against spike antigen mutations and variants of concern where we have seen inconsistent protection from existing FDA-approved vaccines.
Finally, we believe there are also logistical advantages of CM04S1, including minimal to no need for extreme refrigeration/frozen state – our technology allows for “freeze-dried” delivery - which GeoVax sees as a major advantage in many parts of the world. In addition, in regions of the world where Mpox remains a threat, CM04S1 also prevents Mpox (“Monkeypox”) as well as Spox (“Smallpox”), something that isn’t the case for any of the first-generation mRNA vaccines.

GeoVax has been focused on advancing GEO-CM04S1 in a Phase 2 study (ClinicalTrials.gov ID: NCT04977024), investigating GEO-CM04S1 as a primary vaccine for immunocompromised cancer patients. This study pits GEO-CM04S1 head-to-head versus Pfizer’s mRNA vaccine (Comirnaty®), with the primary goal of showing increased immune response and subsequent protection from Covid-19 in patients with blood cancer who have received stem cell transplant or cellular therapy. If successful, GeoVax could be in a position to seek Emergency Use Authorization for GEO-CM04S1 in this population in late 2023 or early 2024. We remind investors that GeoVax’s MVA platform was originally developed as a smallpox vaccine specifically for use in patients with some level of a compromised immune system.
GeoVax is also investigating GEO-CM04S1 in a second Phase 2 (ClinicalTrials.gov ID: NCT04639466) as a booster for healthy patients who have previously received either the Pfizer or Moderna vaccine as their initial inoculation. Similar to the study above, the goal here is to show a superior immune response and subsequent protection. The immunological responses measured throughout the study will include both the level of SARS-CoV-2 neutralizing antibodies and specific T-cell responses.

We are developing GEO-CM02 as a universal vaccine to address evolving SARS-CoV-2 variants. Using our MVA-VLP™ platform, we have developed a design strategy for vaccines expected to induce broader immunity through the inclusion of multiple genetically conserved structural proteins from the target pathogen. The MVA-VLP™ platform is known to induce a balanced humoral (antibody) and cellular (T-cells) response against the multiple encoded immunogens, potentially limiting immune escape against emerging variants.
Expression of the Spike (“S”) protein, nucleocapsid (“N”) protein, and membrane (“M”) proteins by MVA supports the in vivo formation of virus-like particles, or VLPs, which induce both antibody and T-cell responses. The incorporation of sequence-conserved structural and nonstructural proteins can provide targets for T-cell responses to increase the breadth and function of vaccine-induced immune responses. This strategy provides the basis for generating a universal vaccine with the augmented potential to alleviate the burden of disease caused by circulating coronaviruses.arly this year, the White House announced Project Next Gen, a $5 billion initiative, the follow-up from Operation Warp Speed seeking COVID-19 vaccines with an enhanced breadth of protection against variants and improved durability, particularly being interested in novel vaccine candidates in a clinical trial or capable of entering clinical trials within the next nine months. We believe the CM04S1 is the leading example of the desired next-generation vaccines. We have considerable interest, both domestically and internationally, in participating in our clinical development program. We believe that an opportunity for an expedited regulatory path will likely exist due to our focus on high-risk populations unserved by the current COVID-19 vaccines as well as the monoclonal antibody therapy. We anticipate partnering collaborations and support of worldwide commercialization and distribution

There are an estimated 15 million patients in the U.S. considered immunocompromised and another 240+ million worldwide. Examples of such patients include individuals with blood cancers, autoimmune diseases (e.g., Lupus), HIV-infected, sickle cell anemia patients, those with renal disease, patients on immune-suppressive therapies (e.g., transplant patients), etc. The first-generation vaccines and monoclonal antibody therapies are inadequate for these patients. This is our targeted immunocompromised population in our first Phase 2 study. In immunocompromised patients, CM04S1 is the most advanced COVID-19 vaccine worldwide addressing this population.
The second Phase 2 booster study, which is currently enrolling, will include 60 healthy individuals, 18 years of age or older, that were previously vaccinated with one of the approved SARS-CoV-2 mRNA vaccines. Over 220 million Americans received at least one dose of the first-generation mRNA vaccines. Of those, 69.5 million people are over the age of 60 and are recommended by the U.S. CDC to receive annual boosters to maintain durable immunity. GeoVax believes that the booster population is an even larger market opportunity for CM04S1 than the immunocompromised population.

Ebola, Sudan, and Marburg viruses are the most virulent species of the Filoviridae family. They can cause up to a 90% fatality rate in humans and are epizootic in Central and West Africa, with 29 outbreaks since 1976. The 2013-16 Ebola outbreak caused 28,616 cases and 11,310 deaths (40% fatal). We have demonstrated 100% single-dose protection in preclinical lethal challenge models for our Ebola vaccine and are developing vaccines against Sudan and Marburg, which also have pandemic potential. Our Ebola vaccine has completed efficacy testing in non-human primates and is ready for GMP manufacture and phase 1 human trials.
Lassa fever virus, a member of the Arenaviridae family, causes severe and often fatal hemorrhagic illnesses in an overlapping region with Ebola. In contrast to the unpredictable epidemics of filoviruses, the Lassa virus is endemic in West Africa with an annual incidence of >300,000 infections, resulting in 5,000-10,000 deaths. Data from a recent independent study suggest that the number of annual Lassa Fever cases may be much higher, reaching three million infections and 67,000 deaths, putting as many as 200 million persons at risk. Our initial preclinical studies in rodents for our Lassa Fever vaccine candidate have shown 100% single-dose protection against a lethal challenge composed of multiple strains of Lassa delivered directly into the brain. The study was conducted at the Institute of Human Virology at the University of Maryland School of Medicine in Baltimore. We are currently conducting advanced preclinical testing funded by a grant from the U.S. Department of Defense and being performed in collaboration with USAMRIID and the Geneva Foundation.
Zika virus infection has been linked to an increase in microcephaly in infants and Guillain-Barre syndrome (a neurodegenerative disease) in adults. We have achieved 100% protection of mice when vaccinated with a single dose of our Zika vaccine and exposed to a lethal challenge injected directly into the brain. Our Zika vaccine is based on the NS1 protein of Zika, which is not associated with Antibody-Dependent Enhancement (ADE) of infection, a safety concern for other Zika vaccines under development. Moreover, an NS1-based vaccine has the potential advantage of blocking the transmission of Zika from humans to its mosquito vectors.
Globally, malaria causes 228 million infections and 405,000 deaths annually. Despite decades of vaccine research, vaccine candidates have failed to induce substantial protection (e.g. >50%). Most of these vaccines are based on truncated proteins or VLP proteins targeting a limited number of antigens derived from only one stage of the malaria parasite’s life cycle. Our MVA-VLP multi-antigen malaria vaccine candidates are designed to induce a Th1-biased immune response with durable functional antibodies (IgG1 and IgG3) and CD4+ and CD8+ T cell responses, all hallmarks of an ideal malaria vaccine. We have collaborated with the Burnet Institute, a leading infectious disease research institute in Australia, as well as with Leidos, Inc. (under a contract from USAID Malaria Vaccine Development Program) for the development of a vaccine to prevent both malaria infection and transmission by targeting antigens derived from multiple stages of the parasite’s life cycle.

On Gedeptin® (Advanced Head and Neck Cancers):
- Completion of FDA-funded portion (10 patients) of Phase 2 trial by year-end
- Hold regulatory discussions with the U.S. FDA regarding expanded Phase 2 trial and potential for expedited registration pathway
On GEO-CM04S1 (COVID-19):
- Initiate multisite expansion of Phase 2 booster trial and completed enrollment
- Complete enrollment of the multisite Phase 2 immunocompromised trial
- Initiation of a third Phase 2 trial in patients with Chronic Lymphocytic Leukemia (CLL)
- Hold regulatory discussion with the U.S. FDA regarding an expedited registration pathway (immunocompromised patients)
On GEO-MVA (Mpox; Spox):
- Clarify regulatory registration pathway
- Progress on GEO-MVA Continuous Cell-line Manufacturing

In 2022, GeoVax strengthened its balance sheet, adding $37 million during a time in which many biotech firms were furloughing programs and or people. We feel that our capital development success has reflected investor support and belief in the value growth opportunities underway at the Company. We continue to receive strong interest-related investment capital, which we will evaluate, but we’re focused on executing our 2023 goals, strengthening shareholder value, and achieving critical reporting milestones for our development programs.
As of March 31, 2023, GeoVax held $23.9 million in cash and investments, as compared to $27.6 million at December 31, 2022. The change in cash is reflective of the $3.8 million used in operating activities during the first quarter of the year. There were no significant financing or investing activities during the first quarter. Funding our three ongoing Phase 2 clinical programs is the most important use of our cash and our top financial priority. Our cash runway is sufficient to fund these programs through the milestones expected to occur over the course of this year.

Our outstanding common shares now stand at 26.4 million.

There are four analysts that follow GeoVax. As of June 2023, two analysts rate the stock as Strong Buy and two analysts rate the stock as Buy.
The average analyst recommendation is Buy, and the average price target is $5.75 per share (range: $3.00 to $8.00).
GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.
Further information on our risk factors is contained in our periodic reports on Form 10-Q and Form 10-K that we have filed and will file with the SEC. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our results to differ may emerge from time to time, and it is impossible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.










