Turn on the TV or flip through social media and the coronavirus outbreak in Wuhan, China dominates the headlines. The situation is continually evolving, with seemingly exponential increases in the numbers each day with rapid expansion of infected populations beyond China. The vast majority of these cases appear to be mild to moderate in nature. However, in about 15-20% of patients, symptoms can become severe and include pneumonia, acute respiratory syndrome, and even death.
Click here to view updated data on the COVID-19 outbreak
The World Health Organization WHO) declared COVID-19 a Global Health Emergency on January 30th. The U.S. Centers for Disease Control and Prevention (CDC) followed with its own Public Health Emergency warning on January 31st.
We’ve known about the coronavirus for decades. Coronaviruses (CoV) are actually a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronaviruses are zoonotic, meaning they are transmitted between animals and people. This 2019 novel coronavirus (COVID-19) is a new strain that has not been previously identified in humans.
Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. The incubation period is averaging around five days, but can be as long as two weeks. This is the period during which an infected person may transmit the virus before they even see any symptoms. This is part of the reason why the COVID-19 strain seems far more infectious than previous strains. That’s the scary part, and it begs the question:
Why aren’t we more prepared for these global outbreaks?
The WHO and the CDC certainly are not taking the COVID-19 outbreak lightly. On February 5th, The WHO updated its Strategic Preparedness and Response Plan (SPRP) to earmark $675 million in funding to combat the global epidemic. The Bill & Melinda Gates Foundation pledged an additional $110 million in funding to improve detection, isolation, and treatment for at-risk populations in Africa and South Asia. The funding includes efforts to accelerate the development of vaccines, drugs, and diagnostics specifically for COVID-19.
But with many experts now saying this strain of the virus is likely to become seasonal, the consensus is that by April in the U.S., this will all be out of sight and out of mind. Like the Flu, which has already killed five-times the number of people in the U.S. as COVID-19 in China, we generally just accept that there will be deaths. This attitude needs to change.
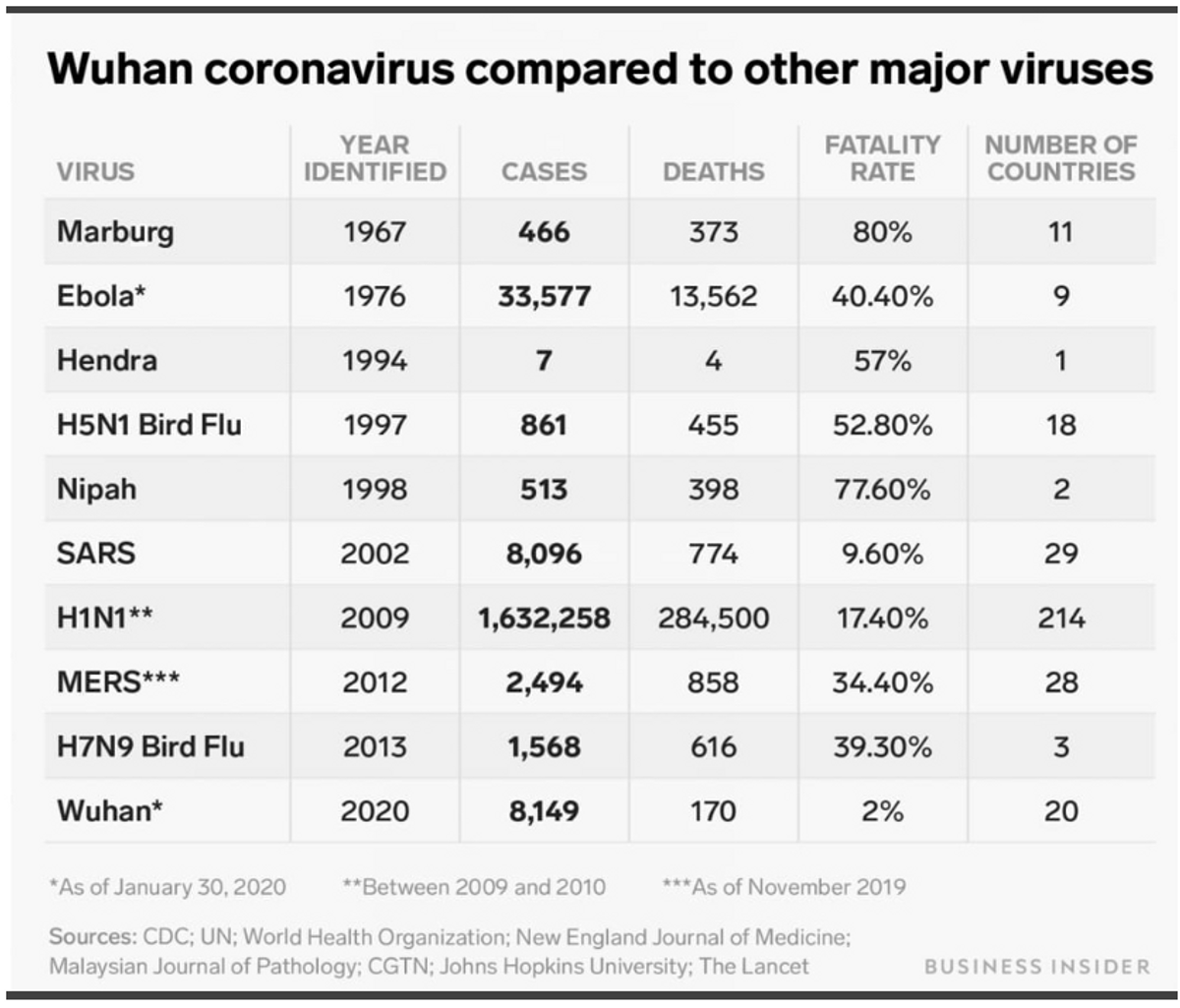
Perhaps the general public's indifference towards the COVID-19 outbreak is because it’s in China or the fatality rate is only around 2%. Roughly 80% of those who died were over the age of 60, and 75% had pre-existing conditions such as cardiovascular disease and diabetes. This is significantly lower than previous global outbreaks. SARS killed one-in-ten, MERS and Ebola around one-in-three, and Marburg a whopping four-out-of-five.
Still, with all this available funding, pharmaceutical and biotechnology companies should have an economic interest in developing preventive vaccines and therapeutics to treat these rampant infectious diseases. Several large pharmaceutical companies have already made public statements about the uses of existing medications to treat coronavirus. AbbVie has made AIDS drug Aluvia® (lopinavir/ritonavir) available to the Chinese health authorities per request. J&J noted it will leverage its AdVac® and PER.C6® technologies that provide the ability to rapidly upscale production of the optimal vaccine candidate. And Gilead said it is experimenting with remdesivir, an experimental Ebola drug, to see if there is utility in combating COVID-19. Remdesivir has demonstrated some previous success in MERS and SARS.
Of the above strategies, Gilead’s remdesivir, seems the most attractive. Remdesivir is a small molecule adenosine analog with demonstrated efficacy in non-human private models of Ebola infection. However, there’s also evidence that remdesivir also works in two previous coronavirus outbreaks, SARS and MERS, and the newly discovered Nipah virus. Remdesivir has what organizations like the WHO and the NIH and look for when they allocate funding - one molecule with the potential to treat several different pathogens. Although, remdesivir is not a platform, it’s just a single asset.
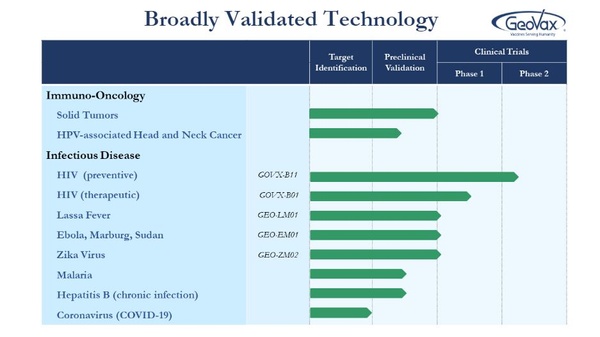
What seems a better long-term strategy is to fund vaccine development platforms that have demonstrated preclinical safety, immunogenicity, and protection in multiple diseases so that organizations like the WHO, the CDC, the NIH, the Biomedical Advanced Research and Development Authority (BARDA), and Coalition for Epidemic Preparedness Innovations (CEPI) can access flexible vaccine platforms for rapid response to outbreaks and emergence of such infectious threats. One such company that might offer such a validated vaccine platform solution is Atlanta-based GeoVax Labs, Inc. (GOVX).
GeoVax has developed a novel Modified Vaccinia Ankara (MVA) Virus-Like Particle (VLP) platform technology that has been demonstrated safe and effective and has shown the ability to induce rapid immunity and protection against a wide range of pathogens in animal models of Ebola, Marburg, Lassa, and Zika. The GV-MVA-VLP™ platform technology is built on a vector system that is optimized for high expression and stabilization during manufacture.
Vaccines built on the GeoVax platform offer several important attributes:
- Safety in all target populations - The safety of previous MVA has been demonstrated in over 120,000 subjects, including the immuno-compromised and the safety of the GeoVax vaccine technology has been demonstrated in ~500 subjects.
- Rapid elicitation of protection - MVA-VLP vaccines elicit protective immunity in animal models of Ebola, Lassa, and Zika after a single dose in under two weeks. MVA vaccines can also be used simultaneously primed with DNA, protein, or peptide vaccines for an enhanced effect.
- Powerful and durability response - MVA-VLP vaccines have a high “carrying capacity” to express multiple viral antigens. Constructs have shown the ability to elicit both humoral (antibody) and cellular (T-cell) responses with truly exceptional durability without the need for adjuvants.
- Favorable stability and logistics - MVA is stable in both liquid and lyophilized dosage forms, allowing for suitable long-term storage at refrigerator temperatures. MVA can reliably be manufactured in Chicken Embryo Fibroblasts (CEFs) or novel continuous cell lines (e.g.AGE1.CR cells) that support scalability as well as greater process consistency and efficiency.
The GeoVax vaccine for HIV (GOVX-B11) has demonstrated outstanding safety, tolerability, and immunogenicity in six human trials and is currently in multiple human trials working toward a “functional cure.” The company has also designed, vaccine constructs and analyzed the protective efficacy against numerous emerging infections with epidemic potential. Preclinical studies have been completed for vaccines against Ebola virus (MVA-EBOV), Marburg virus (MVA-MARV), Lassa virus (MVA-LASV) and Zika virus (MVA-NS1).
Below is a summary of the data generated to date.
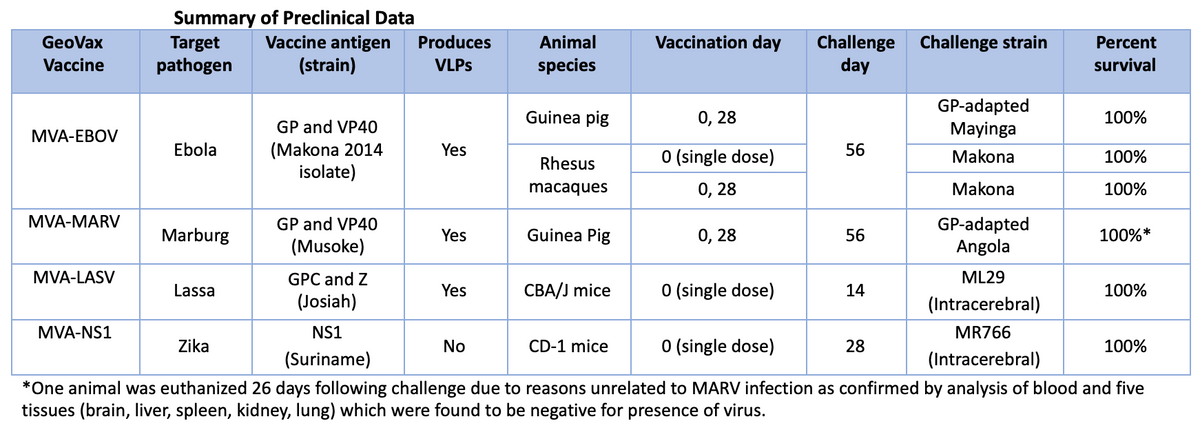
The company has also designed, vaccine constructs and analyzed the protective efficacy against numerous emerging infections with epidemic potential. Preclinical studies have been completed for vaccines against Ebola virus (MVA-EBOV), Marburg virus (MVA-MARV), Lassa virus (MVA-LASV) and Zika virus (MVA-NS1).
Below is a summary of the data generated to date.
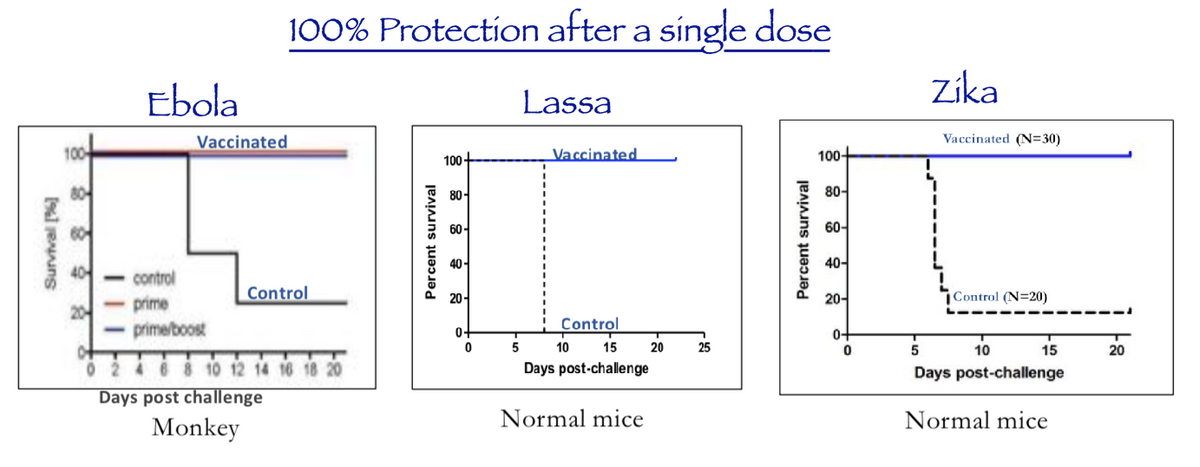
These are impressive results and now is the ideal time to start advancing these candidates into human clinical testing. Emerging epidemics like the one we are seeing now with COVID-19 are frightening and it is clear we need to do more to prepare for these outbreaks in the future. The 2019 novel Coronavirus (COVID-19) has not been overly fatal, but it is still wreaking havoc on the Chinese economy and it clearly shows that we are not prepared for potentially more dangerous infectious diseases that may arrive.
Recently, GeoVax announced that it is working with BravoVax, a vaccine developer based in Wuhan, China, the epicenter of the COVID-19 outbreak. In this collaborative effort, vaccine candidates to prevent COVID-19 are being designed based upon the GV-MVA-VLP™ technology and expertise. Both GeoVax and BravoVax are actively pursuing funding for parallel efforts related to pre-clinical testing, focused on advancing a clinical candidate for human trials.
Story on Atlanta's NPR News: Atlanta Biotech Company Is Working On A Coronavirus Vaccine
Developing nations are often the ones hit the hardest by outbreaks. A 2016 study conducted by Johns Hopkins University and published in Health Affairs (Ozawa S et al., 2016) found that for every dollar invested in vaccination in the world’s 94 lowest-income countries, $16 are expected to be saved in healthcare costs, lost wages and lost productivity due to illness and death.
Organizations like the WHO, U.S. CDC, NIH, BARDA, and CEPI are providing emergency funding and companies like GeoVax are in a position to advance vaccine candidates in a timely manner.
Cautionary Language Regarding Forward-Looking Statements. This material contains forward-looking statements. Forward-looking statements relate to future events. GeoVax generally identifies forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar words, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements regarding our management’s expectations, hopes, beliefs, intentions or strategies regarding the future, such as our expectations as to actions taken by our primary investors with respect to conversion of our preferred stock into common stock and the sale of such common stock; future publication of information on changes in our outstanding common stock; the initiation, timing, progress and results of our preclinical and clinical trials, research and development programs; our liquidity and our expectations regarding our needs for and ability to raise additional capital; and other factors discussed elsewhere in these materials. GeoVax undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.