ATLANTA, GA, November 7, 2019 – GeoVax Labs, Inc. (OTCQB: GOVX), a biotechnology company developing human immunotherapies and vaccines, today announced its financial results for the quarter ended September 30, 2019 and provided an update on its corporate development progress.
David A. Dodd, GeoVax President and CEO, commented, “We continue making progress in several product development areas despite our constrained capital resources. With the recent selling pressure inflicted upon our common stock as holders of our convertible preferred stock are liquidating their position, it’s easy for investors to lose sight of the underlying value of our company, and the promise of our technology. With that said, we are providing this comprehensive review of our various development programs.”
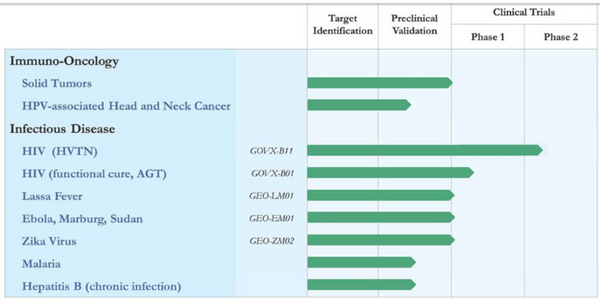
- Cancer Immunotherapy – We began our work in this area with a single program (tumor-associated MUC1 vaccines) supported by collaborations with the University of Pittsburgh and ViaMune, Inc. and expanded to a second tumor-associated antigen (Cyclin B1) in collaboration with Vaxeal Holding SA. During 2018, we added additional collaborations to expand our footprint in this space – most notably with Emory University, for HPV-related head and neck cancers, and with Leidos, Inc. to evaluate delivery of Leidos’ novel PD-1 checkpoint inhibitors with our MVA-VLP platform for multiple immunotherapeutic candidates. To date, in humanized mouse models evaluating our MUC-1 vaccine, we have demonstrated significant tumor reduction, as well as tumor growth prevention.
Immuno-oncology represents an area of significant medical need and our results thus far have been promising. We believe developing our programs in this area to be a key component for strengthening the valuation of GeoVax and providing future value growth opportunity. In order to facilitate targeted funding of these programs, we are forming a separate subsidiary to house our oncology assets. In addition, discussions are underway with other oncology companies about potentially bringing in clinical-stage development programs that would be synergistic with our technology, as well as discussions with private investors regarding capital investment in the oncology subsidiary. The concept is that all of our oncology work would be performed within the new subsidiary, with additional joint-development and licensing opportunities.
- HIV “Functional Cure” Immunotherapy – We are participating in a planned clinical trial led by researchers at American Gene Technologies (AGT) (www.americangene.com), to develop a therapy aimed at eliminating HIV from infected people. On October 18, AGT announced the submission of an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for its lead HIV program, AGT103-T, a lentiviral vector-based gene therapy. Upon clearance by the FDA, this IND will allow AGT to initiate a Phase 1 clinical trial that will investigate the safety of AGT103-T in humans, measure key biomarkers, and explore surrogate markers of efficacy. AGT expects to begin recruiting patients for the Phase 1 study in January 2020. GeoVax will provide our novel MVA-VLP HIV vaccine (MVA62B) for evaluation in an arm of the clinical trial in combination with AGT103-T. T cells obtained from vaccinated individuals will be reprogrammed by AGT’s lentivirus vector and infused back into the study participants as a therapeutic cell product. MVA62B is the boosting component for GeoVax’s preventive HIV vaccine (GOVX-B11) which has completed a Phase 2a clinical trial.
- HIV Preventive Vaccine – We are planning for a new Phase 1 human clinical trial with operational support from the HIV Vaccine Trials Network (HVTN) and funding from the National Institute of Allergy and Infectious Diseases (NIAID). We expect that HVTN will begin the next study (designated HVTN 132) in mid-2020.
In July, at the 10th International AIDS Society Conference on HIV Science in Mexico City, Harriet L. Robinson, PhD, GeoVax’s Chief Scientific Officer Emeritus and Director of our HIV Vaccine program, delivered an oral presentation entitled, Protein-Supplemented DNA/MVA Vaccines: Preclinical Immunogenicity and Protection for Transmitted/Founder and CD4-induced gp120 Proteins. In her talk, Dr. Robinson reported on studies in 72 rhesus macaques evaluating the effects of the addition of gp120 proteins to the MVA boosts of a DNA prime-MVA boost vaccine regimen. The gp120 boosts consisted of a gp120 protein from a transmitted/founder virus (to present the receptor-binding form of gp120) and a CD4 receptor-induced gp120 protein (to present the fusion-intermediate form of gp120). Both intramuscular and subcutaneous delivery of the boosts were tested. The gp120 boosts enhanced the magnitude and breadth of binding antibodies (bAbs) to gp120. The CD4-induced, but not the transmitted/founder gp120, enhanced bAbs for the V1V2 region of gp120, a specificity that correlated with reduced infection in the partially successful RV144 HIV vaccine trial in Thailand. The subcutaneous, but not the intramuscular injections, for the gp120 proteins reduced the level of post challenge infection.
The human clinical trial (HVTN 132) being planned in conjunction with NIAID and HVTN is similar in design as the preclinical study discussed by Dr. Robinson in her IAS presentation (GOVX-B11 DNA prime and MVA boost vaccine with or without protein boosts).
- Malaria Vaccine – In March, we announced our collaboration with Leidos, Inc. to develop malaria vaccine candidates. Our work is being supported by a contract to Leidos from the United States Agency for International Development (USAID) Malaria Vaccine Development Program (MVDP). Leidos has been tasked by USAID to advance promising vaccine candidates against P. falciparum malaria and selected the GeoVax MVA-VLP platform to be a part of this development effort.
Currently there is a shortage of malaria vaccine candidates that can offer the high efficacy rates (e.g. >75%) set by the World Health Organization (WHO) as a requirement for the second-generation malaria vaccines. Although protein-derived vaccines can deliver multiple antigens in immunogenic VLP conformation, they hardly produce a balanced functional cellular immune response needed to confer a high protection. In contrast, vectored-derived live vaccines are capable of producing the appropriate balanced immune responses, but they suffer from limitations in delivering the required number of transgenes needed to protect against all stages of malaria parasite. GeoVax’s MVA-VLP platform can potentially overcome both limitations of antigen conformation and transgene capacity by delivering multiple transgenes (e.g. from parasite’s liver stage, blood stage and mosquito stage) in the form of VLPs delivered in vivo.
Our collaboration with Leidos complements our ongoing malaria vaccine development project with Burnet Institute in Australia and offers multiple opportunities for success.
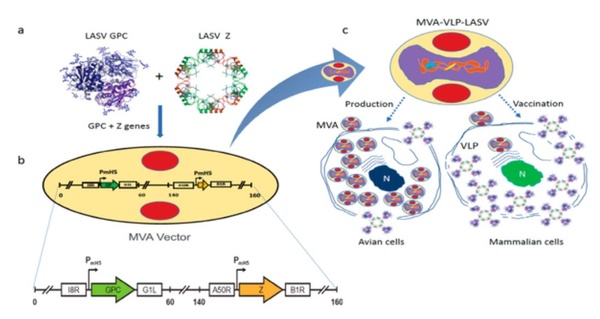
- Lassa Fever Vaccine – In September, we announced the publication of an article entitled “A Single Dose of Modified Vaccinia Ankara Expressing Lassa Virus-like Particles Protects Mice from Lethal Intra-cerebral Virus Challenge.” The paper appears in the open access journal Pathogens published by MDPI, based in Basel, Switzerland, and can be viewed at https://www.mdpi.com/2076-0817/8/3/133. The paper published in Pathogens reports research showing that a single intramuscular (IM) dose of our Lassa fever vaccine (GEO-LM01) provided 100% protection in mice challenged with a lethal dose of ML29 (a Mopeia/Lassa reassortant virus) delivered directly into the brain. This is the first report of an MVA-based Lassa vaccine showing full protection against a lethal challenge.
During 2019, we have continued our progress in this program with grant support from the U.S. Department of Defense to advance our vaccine through nonhuman primate testing and manufacturing process development in preparation for human clinical trials. There currently is approximately $1.8M in committed grant funds supporting this program going forward.
- Solid-Dose Needle-Free Vaccine Delivery– Earlier this year, we announced a collaboration with Enesi Pharma to develop solid-dose needle-free vaccine formulations utilizing our MVA-VLP vaccine platform in combination with Enesi’s ImplaVax® device and formulation technology. Our collaboration contemplates development of solid-dose vaccines for multiple infectious diseases and evaluation of the potential to generate improved vaccine responses with simplified administration and reduced storage and distribution costs. The combination of our thermostable vaccines with Enesi’s needle-free device is a natural fit and we think there is significant scientific rationale for expecting success, especially in resource constrained countries where cold chain storage is an issue.
The Bill and Melinda Gates Foundation recently awarded Enesi a grant for development of solid-dose vaccines for Measles and Rubella, thereby recognizing the strong scientific foundation of Enesi’s technology and its potential to benefit world health. We are delighted to be collaborating with Enesi and look forward to sharing more information about our disease targets and study results in the near future.
- Emerging Infectious Disease Vaccines – Hemorrhagic fever viruses are a continuing health threat from both the endemic and bioterrorism perspectives, exemplified by the ongoing Ebola outbreak in the Democratic Republic of the Congo. In addition to our Lassa vaccine discussed above, our Ebola and Marburg vaccines have each demonstrated 100% preclinical protection, and have economical cold chain requirements, all critical attributes for such vaccines and their implementation. Likewise, Zika virus remains a recurrent threat to populations in tropical regions and our Zika vaccine has demonstrated 100% preclinical protection. We have recently offered these vaccines (beginning with Ebola) to public health agencies worldwide, contingent on their funding the advancement into clinical development and human use.
Mr. Dodd continued, “This update is intended to convey to our shareholders, potential investors, and other stakeholders the continued progress in the underlying value of our Company and development programs. Our common stock has been significantly diluted through conversions of preferred stock, causing our outstanding common share count to rise from 95.6 million shares at September 30 to currently over 426 million shares. We expect this to continue until the preferred stock investors have exited their position, which currently stands at approximately $2.5 million. Due to the resultant sharp decline in our stock price and rapid increase in our outstanding shares, we will not have sufficient authorized common shares to meet our obligations to the preferred stock investors. This situation unfortunately leaves us with limited choices but to seek stockholder approval for another reverse stock split to remedy the lack of authorized shares, which may also increase our share price and allow us to maintain our listing on the OTCQB Stock Market.
“But these issues do not detract from the potential value of our company and the promise of our technology; our current financing situation is challenging, but not insurmountable. I am a firm believer in our technology, our people, and our collaborators, and I have confidence that we will succeed. With the clean-up of our capital structure, implementation and progress of our oncology subsidiary, and continued advancements in our infectious disease programs, we will be much better positioned to secure additional capital on reasonable terms and increase long-term shareholder value.”
Financial Review
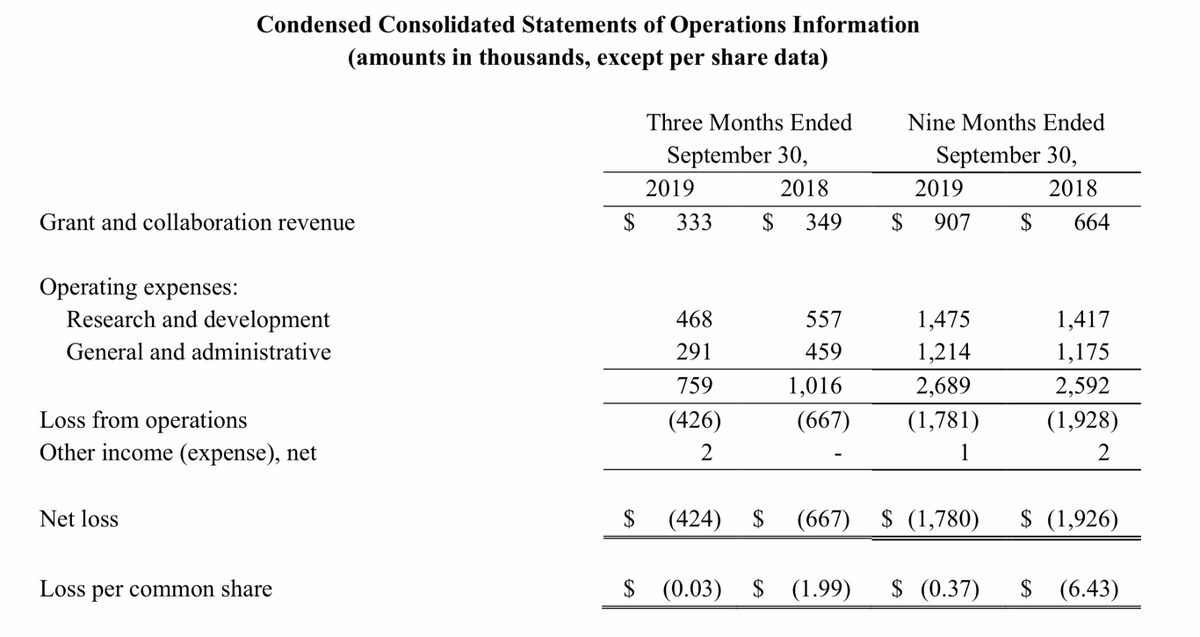
GeoVax reported a net loss of $424,434 ($0.03 per share) for the three months ended September 30, 2019, compared to $666,893 ($1.99 per share) for the same period in 2018. For the nine months ended September 30, 2019, the Company’s net loss was $1,780,036 ($0.37 per share) as compared to $1,925,749 ($6.43 per share) in 2018.
The Company reported grant and collaboration revenues of $333,209 and $907,382 for the three-month and nine-month periods of 2019, respectively, as compared to $349,344 and $663,908 reported for the comparable periods of 2018. As of September 30, 2019, there is $1,835,225 in approved grant funds remaining and available for use.
Research and development (R&D) expenses were $467,674 and $1,474,619 for the three-month and nine- month periods of 2019, respectively, as compared to $557,696 and $1,416,892 for the comparable periods of 2018. Fluctuations in R&D expenses from period to period are primarily attributable to the timing of expenditures related to government grants. General and administrative (G&A) expenses were $291,475 and $1,214,189 for the three-month and nine-month periods of 2019, respectively, as compared to $458,974 and $1,175,399 for the comparable periods of 2018.
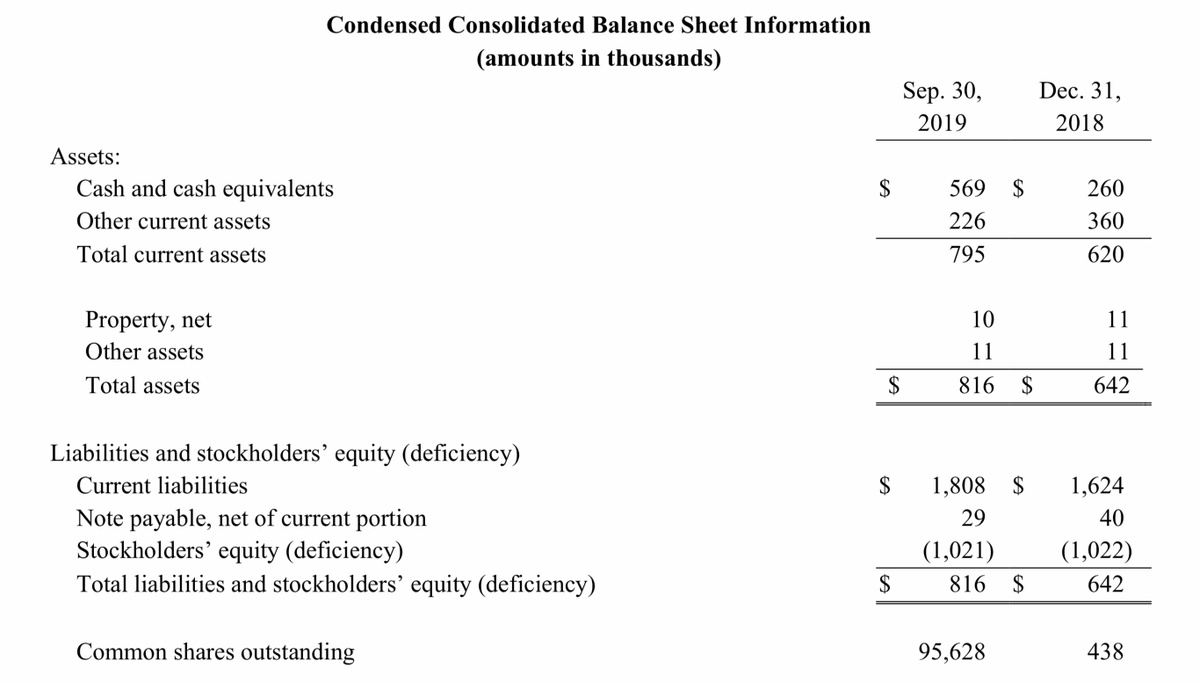
GeoVax reported cash balances of $569,359 at September 30, 2019, as compared to $259,701 at December 31, 2018. Further information concerning the Company’s financial position and results of operations are included in its Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission.
About GeoVax
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing human vaccines against infectious diseases and cancer using a novel patented Modified Vaccinia Ankara-Virus Like Particle (MVA-VLP) based vaccine platform. On this platform, MVA, a large virus capable of carrying several vaccine antigens, expresses proteins that assemble into VLP immunogens within (in vivo) the person receiving the vaccine. The production of VLPs in the person being vaccinated mimics virus production in a natural infection, stimulating both the humoral and cellular arms of the immune system to recognize, prevent, and control the target infection. The MVA-VLP derived vaccines elicit durable immune responses in the host similar to a live-attenuated virus, while providing the safety characteristics of a replication-defective vector.
GeoVax’s current development programs are focused on preventive vaccines against HIV, Zika Virus, hemorrhagic fever viruses (Ebola, Sudan, Marburg, and Lassa), and malaria, as well as therapeutic vaccines against chronic Hepatitis B infections and multiple cancers. The Company has designed a preventive HIV vaccine candidate to protect against the subtype of HIV prevalent in the larger commercial markets of the Americas, Western Europe, Japan, and Australia; this program is currently undergoing human clinical trials managed by the HIV Vaccine Trials Network (HVTN) with the support of the National Institutes of Health (NIH). GeoVax’s HIV vaccine is also part of collaborative efforts to develop an immunotherapy as a functional cure for HIV. For more information, visit www.geovax.com.
Forward-Looking Statements
Certain statements in this document are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act. These statements are based on management's current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: any of GeoVax’s collaborative efforts will be successful, GeoVax can develop and manufacture its vaccines with the desired characteristics in a timely manner, GeoVax's vaccines will be safe for human use, GeoVax's vaccines will effectively prevent targeted infections in humans, GeoVax’s vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete vaccine development, there is development of competitive products that may be more effective or easier to use than GeoVax's products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control. GeoVax assumes no obligation to update these forward-looking statements and does not intend to do so. More information about these factors is contained in GeoVax's filings with the Securities and Exchange Commission including those set forth at "Risk Factors" in GeoVax's Form 10-K.
FINANCIAL TABLES FOLLOW
GEOVAX LABS, INC.
Condensed Consolidated Statements of Operations Information
(amounts in thousands, except per share data)